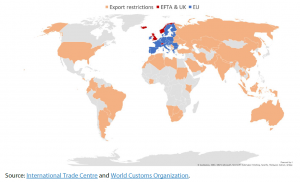
Responding to the shortage of medical products to tackle the coronavirus pandemic, a large number of countries implemented either de facto or legal restrictions on exports of medical products. On 15 March, the European Commission itself introduced Implementing Regulation (2020/402) on authorisation requirements for exports of personal protective equipment outside the European Union (EU); it came into effect on 21 March.
The coronavirus crisis has revealed the dependence of the EU on imports of medical products from non-EU countries. EU Member States have therefore been taking emergency initiatives in collaboration with the EU to produce and distribute medical products, especially personal protection and artificial respiratory equipment, as existing producers have been unable to meet the skyrocketing worldwide demand. This raises the question of the need for the EU to enhance its production independence in the future.







Be the first to write a comment.